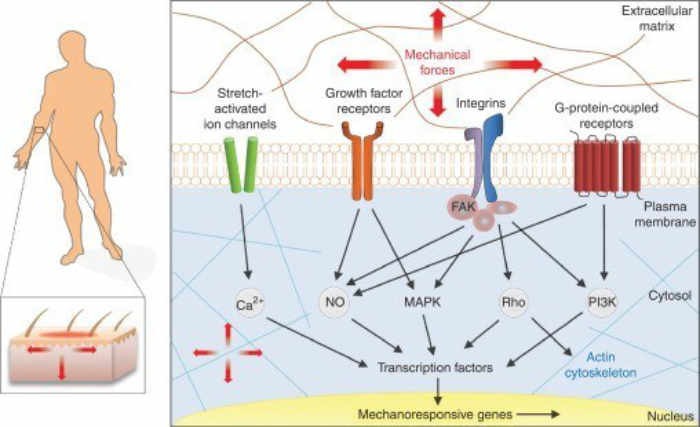
The above diagram has an important take-home message, which is that physical therapy can accomplish many of the same tasks as stem cells. What we have learned is that therapy, represented by “mechanical forces”, causes the formation of growth factors, and awakens “sleeping” genes to turn on and accomplish repair. This is why therapy after a regenerative cell procedure is so important.
We recently co-authored an article published in the October 2018 issue of the “Strength and Conditioning Journal”. The lead author is Dr. Morey Kolber, a professor of Physical Therapy at Nova Southern University in Ft. Lauderdale Florida. In the article, we looked at a number of parameters for treating patients after they received a regenerative medicine procedure consisting of a PRP (Platelet Rich Plasma) injection. The main emphasis of the article concerned PRP injections but I will take a bit of poetic license and include bone marrow and adipose injections since there should be no significant differences when using these products.
The purpose of this blog is to highlight some of the important takeaways from the article. We are all aware of the concept that exercise is important post injection. At the same time, we dispel some common myths in the regenerative world such as the use of ice and NSAIDS following injections. At the end of the blog a copy of the published article is included.
On the day of a procedure our patients are instructed to begin moving the treated area right away even though they were in pain. I would jokingly tell them that I would give them a Voodoo doll with my likeness that they could stick pins into since they would be a great deal of pain. Furthermore, I believe in a full flare PRP which many times increases pain but that is the topic of another blog.
As my patients are aware I am a firm believer in physical therapy (PT). Perhaps this is a belief acquired from being a practicing orthopedic surgeon for many years where I would always attempt to get the patient into therapy ASAP. Some patients thought I was being too harsh on them since they were still hurting and felt I should let them recover before starting PT. However, I was acutely aware that patients put into Physical Therapy sooner rather than later typically got better quicker and seemed to have a better overall result. I was not exactly sure why until I acquired a firm understanding of regenerative medicine and did some research on what PT actually does. For his part, Dr. Kolber has certainly reinforced and consolidated this knowledge.
When I lecture, I often use a slide which demonstrates the essence of regenerative medicine namely, “Cells not Doctors Heal Patients”. I am now convinced that PT is absolutely in step with that concept. PT can have some profound biochemical effects on individual cells, changing their environment, which can result in healing.
There are essentially three main phases of healing following a regenerative medicine procedure; the acute phase followed by the proliferative phase and finally the remodeling phase.
In the first three days following the procedure, pain is usually the overriding factor. This phase includes the acute inflammatory phase. Here, we encourage the extent of motion the patient is able to tolerate. We encourage such motion since this often seems to make the acute inflammatory phase run its course quicker. During this phase, we encourage cryotherapy (ice) and a variety of analgesic type compounds including NSAIDS (non-steroidal anti-inflammatory medications). The use of cryotherapy and NSAIDS may raise some eyebrows. This is where some of the “Urban Legends” seem to come into play. There is a popular perception that cryotherapy may hinder PRP outcomes in that ice may reduce platelet activation and interfere with the intended inflammatory cascade after a PRP. However, there are no studies that have demonstrated an inferior result from ice and studies have used ice after a PRP injection with positive outcomes. Thus, there is no conclusive evidence to preclude use of cryotherapy.
The other “Legend” in acute phase is the use of NSAIDS. I do not wish to encourage the use of NSAIDS since we are well aware of some of the risks of these drugs. However, on a short-term basis in the appropriate patient, they can be quite beneficial. A review of the literature on NSAIDS reveals some interesting facts. In one study, NSAID consumption was associated with increased muscle cell activation compared with placebo, suggesting that NSAIDs may have a different effect on injured versus healthy muscle. Although evidence supports the premise that nonselective NSAIDs (e.g., naproxen) may interfere with platelet aggregation, growth factor levels in PRP are not altered.
After reviewing the literature, we can conclude that NSAID use does not compromise growth factor release and is more likely to have a deleterious effect on healthy structures as opposed to pathological tissues. Moreover, other medication classes such as acetaminophen and select opioid drugs may negatively influence post-exercise protein synthesis and produce in-vitro chondrotoxicity respectively. Given the potential for NSAIDs to reduce pain and inflammation and the potential of other medication classes to negatively influence cells, it seems there is little downside to intermittent, low-dose use in the early acute phase. The attaching article goes into this in more detail.
The next phase of post-operative care is the early subacute phase. This typically involves days 4-14. In this phase, stretching and isometrics are stressed. Isometric exercises are a type of strength training in which the joint angle and muscle length do not change during contraction. Isometrics are done in static positions, rather than being dynamic through a range of motion. Activities such as cycling, deep-water running or water walking, and upper-body ergometry are introduced and advanced based on tolerance. Ergometry is a science that measures the amount of physical work done by the body. In cases where a PRP injection is administered to treat Osteoarthritis, progressive and repetitive loading of the joint is encouraged. This seems to support the old adage, “motion is lotion”.
The next phase is what we call the sub-acute phase. This usually found in weeks 3-6. The objective at this phase is to ensure that the co-introduction of mechanical forces is a primary component of the management strategy. The introduction of new exercises and advancement of previously prescribed activities are associated with regenerative changes in pathological tissue, up-regulation of growth factors, and stimulation of cellular tissues. This is one of the overarching goals of the post-operative care.
The last phase is what we call the remodeling phase. This usually occurs after week 7. It is at this time when more advanced loading is initiated. What we have become acutely aware of is the fact that exercise is so important for a successful result. On the surface, this makes perfect sense yet one many have difficulty explaining how the benefits of physical therapy are actually produced. What is of major significance here is the fact that we are actually getting the up-regulation of growth factors when the patient is exercising. Progressive mechanical loading (referred to as mechanotherapy) has been shown to produce a favorable anabolic environment and promote tissue regeneration. This is a very profound observation. We are actually getting growth factors similar to what is found in a PRP or stem cell product by performing mechanical loading exercises. This refers to the field of mechanobiology which fosters a process known as mechanotransduction.
Mechanotransduction is the mechanism by which cells convert mechanical stimuli into cellular responses to a variety of mechanical loads. Cells are sensitive to forces such as shear, tension, and compression, and they respond accordingly through cellular proliferation, migration, tissue repair, altered metabolism, and even stem cell differentiation and maturation. Thus, mechanical stresses can influence genetic expression and cellular behavior. This is an area of molecular biology undergoing rapid exploration and discovery.
In mechanotransduction, each muscle, tendon, and bone are composed of cells that are linked together through an extracellular matrix. Most cells release materials into the extracellular space, creating a complex meshwork of proteins and carbohydrates called the extracellular matrix (ECM) A major component of the extracellular matrix is the protein, collagen. The extracellular matrix is directly connected to the cells it surrounds. Cells adhere to these extracellular matrix scaffolds (composed of collagen, glycoproteins, and proteoglycans) through the binding of specific receptors of the cell surface. These cell surface receptors are called Integrins. Integrins are crucially important because they are the main receptor proteins that cells use to both bind to and respond to the extracellular matrix.
Integrins span the cell surface membrane and communicate signals or stress from the external to the internal environment. Essentially, integrins serve as mechanoreceptors. They are the first molecules on the cell surface to sense a mechanical signal (e.g., compression, tension, etc.) and transmit it across the membrane. These signals can alter gene expression, protein synthesis, and metabolism in a manner similar to hormones, growth factors, and cytokines. Mechanotransduction follows a sequence of 3 steps, which include mechanocoupling, cell-to-cell communication (passing of loading message from one area of tendon to another), and a cellular response such as collagen synthesis or chondrogenesis. This is similar to the methods that stem cells use to accomplish some goals. The following diagram illustrates this process:
Although this diagram seems complicated it gives us the essence of Mechanotransduction. A stress is applied to the membrane and certain growth factors get activated and cause production of various reparative compounds.
In summary, for mechanotransduction to occur, a load is applied to the region of interest. This load, sensed by the integrins, is communicated to the cell where the DNA and cytoplasmic elements are located. When the signal of a “load” is received at the cell, a message is communicated to the cell’s nucleus (DNA). Once the nucleus receives the signal, the messenger RNA transcribes the message and shuttles it to the cytoplasm where it is translated into a regenerative response such as collagen synthesis and growth factors such as IGF-1 which is very important in repair of tissue. These growth factors support what are called anabolic activities. These are activities which support repair.
We can see the extreme importance of exercises especially when performed by a therapist who has a knowledge of not just exercises but the biochemical and cellular ramifications of exercise in helping to cause repair of tissue. Regarding anabolic hormones, growth factors, and cytokines, isometric training has been shown to produce increases in growth hormone, testosterone, and IGF (IGF1Ea and MGF) and decreases in myostatin. As we can see Physical Therapy has been and will always remain an integral part in any regenerative medicine procedure.
Thanks, Dr. Purita
December 07, 2018
Below is the published article.

Platelet Rich Plasma: Postprocedural Considerations for the Sports Medicine Professional
Morey J. Kolber, PT, PhD, CSCS*D,1 Joseph Purita, MD,2 Christian Paulus, Dr. med,3 Jeremy A. Carreno,2 and William J. Hanney, DPT, PhD, ATC, CSCS4
1. Department of Physical Therapy, Nova Southeastern University, Fort Lauderdale, Florida;
2. Institute of Regenerative Medicine, Boca Raton, Florida;
3. American Academy of Regenerative Medicine, Lakewood, Colorado;
4. Doctor of Physical Therapy Program, Department of Health Professions, University of Central Florida, Orlando, Florida.

COLUMN EDITOR: Ben Reuter, PhD, CSCS*D, ATC
Sports Medicine and Rehabilitation
The Sports Medicine and Rehabilitation Column provides practical information on the role of rehabilitation and flexibility on both performance and the modification of injury risk.
ABSTRACT
OWING TO A GROWING INTEREST IN TREATMENTS THAT USE THE BODY’S INNATE HEALING MECHANISMS, SPORTS MEDICINE PROFESSIONALS ARE LIKELY TO ENCOUNTER INDIVIDUALS WITH MUSCULOSKELETAL INJURIES WHO RECEIVED PLATELET-RICH PLASMA (PRP). THIS COLUMN PRESENTS STRATEGIES THAT FOSTER RECOVERY AND HARNESS THE REGENERATIVE POTENTIAL OF PRP. EVIDENCE UNDERPINNING THE IMPACT OF LOADING BIOLOGICAL TISSUES IS PRESENTED TO GUIDE SAFE AND EFFICACIOUS EXERCISE PRESCRIPTION. A COMPANION ARTICLE IN THIS ISSUE DISCUSSES THE SCIENCE AND EVIDENCE SURROUNDING PRP.
INTRODUCTION
Platelet-rich plasma (PRP) is an autologous biological treatment that involves processing whole blood to obtain concentrated cells (primarily platelets) that are then injected directly into or in the proximity of a de- generated or injured anatomical struck- ture (e.g., tendon, joint, muscle, and ligament) (64). Owing to an excellent safety profile and a growing body of scientific literature, it is easy to under- stand the widespread interest in PRP among practitioners in the sports medicine community (64,86). Enthusiasm for biological treatments that harness the body’s own healing mechanisms is not limited to the medical community, as media coverage of high-profile athletes who have received PRP for sports injuries has led to a growing public interest (5,35,64). Although information is available to the public, the accuracy and readability of such information may be limited, particularly regarding the internet (35).
Despite a growing body of favorable outcomes–based research, a paucity of evidence exists to identify a consensus approach for the management of patients who have undergone a PRP injection (61,70,80). Nevertheless, professionals charged with managing patients or clients following a procedure should be familiar with relative precautions and understand the need for appropriately incorporating both rest and progressive physical activity into the postprocedural phases of healing. Sports medicine professionals, armed with an understanding of both musculoskeletal disorders and the regulatory elements of tissue loading, are in an ideal position to provide targeted exercise programming that augments the efficacy of PRP.
The purpose of this column is to present considerations that may guide sports medicine and rehabilitation professionals working with patients or clients, hereafter referred to as “individuals,” who have undergone a PRP injection. The term “considerations” is used in lieu of guidelines when discussing management strategies, as conclusive clinical practice guidelines (from an authoritative body) and evidence to steer postprocedural management are currently in the infancy stage (80). This column, grounded in the available albeit limited evidence, covers the range of postprocedural considerations including use of medications, movement or exercise, and appropriate rest. Given the heterogeneity of musculoskeletal injuries, information presented follows general guidelines gleaned from individual research studies as well as the authors’ personal experience treating patients following PRP procedures. Although the content is written for the broad readership, scope of practice should dictate boundaries with these recommendations. Clearance from the physician who per- formed the PRP procedure is an absolute requirement before initiating any type of intervention or exercise programming. It should be recognized that although there are more common diagnoses being treated with PRP, each patient is unique and only those with appropriate licensing to provide such services should perform specific programming.
OVERVIEW OF BASIC SCIENCE AND EVIDENCE
Although the basic science, biological and clinical evidence, as well as procedures are discussed in a companion article in this issue, a brief overview is necessary to establish content fluency.
The natural healing process of soft tissue entails 3 overlapping stages referred to as the acute, proliferative, and remodeling phases. After injury, blood cells arrive at the affected area and trigger numerous responses. Blood cell actions range from inflammation and clotting to the release of signaling molecules that foster repair (5,34). Although the phases of healing ultimately result in repair or regeneration under the desirable microenvironment, one must recognize that different injuries or circumstances may lead to an inadequate, altered, or failed response.
A PRP procedure involves processing one’s own blood (autologous) to pro- duce supraphysiologic concentrations of growth factors, cytokines, and chemokines (5,34). The concentrations produced with PRP are greater than those present through the normal healing process. Specifically, the blood is processed predominantly to isolate platelets, which contain essential growth factors including but not limited to platelet-derived growth factor that works to establish a blood supply through neogenesis (new vessels) and insulin-like growth factor (IGF) that promotes the synthesis, proliferation, and differentiation of cells. The purported benefit from PRP extends beyond growth factors, as tissue heal- ing may be attributed to a myriad of signaling molecules (chemokines and cytokines) that attract stem cells to the injured area and serve to mitigate inflammation (4,5). After processing, the final PRP product is then injected into the injured region or structure with the goal of resolving the inflammatory response and promoting tissue regeneration (64).
The in vitro evidence for PRP is based on coculturing cells in a PRP solution. A clear body of evidence has shown that tenocytes, myoblasts, chondrocytes, and the less differentiated fibro- blasts and adult stem cells respond favorably to PRP coculturing (6,25,52,62,66,88). A systematic review of 8 in vitro studies identified increased cell proliferation and growth factor expression of tenocytes cultured in PRP (6). Furthermore, an in vitro investigation of myoblasts cultured in PRP resulted in increased cell proliferation and differentiation (52,62). Regarding cartilage, PRP has been shown to induce chondrogenesis of mesenchymal stem cells, and studies of osteoarthritic cartilage have indicated that PRP exposure leads to reduced apo- ptosis and increased proliferation of chondrocytes (65,66).
The clinical evidence shows a relatively good safety profile with swelling, erythema, and pain as the primary adverse effects of PRP (16,84). PRP has shown comparable or superior long-term effects to corticosteroid injections and viscosupplementation for improving function and decreasing pain among individuals with various musculoskeletal disorders (osteoarthritis [OA] and tendinopathy) (7,24,31,33,36,75,78,85,87). Much of the evidence in favor of structural healing or repair comes from case series investigations as opposed to larger comparative trials. Given the lack of a comparison group with case series investigations, causation is difficult to conclude. Nevertheless, current research on PRP is conclusive of a noninferiority outcome and a greater safety profile than some of the more common interventions (e.g., corticosteroid injection or opiates). Aside from the cost of PRP, which has been estimated to range from $500 to 1,500 per injection (cost to patient) (86), there seems to be little downside to PRP. A more detailed overview of the evidence underpinning the use of PRP may be found in a companion article in this issue of the Strength and Conditioning Journal.
POSTPROCEDURAL CONSIDERATIONS
Individuals who have received a PRP injection require special consideration with respect to the underlying injury, premorbid activity level, and time interval since the injection. Sports medicine professionals who possess an understanding of the basic and clinical sciences of PRP and musculoskeletal injuries are in an ideal position to guide individuals through the recovery process and return to premorbid activities. Given that individuals may seek the services of a sports medicine or rehabilitation professional at various stages after a PRP injection, an awareness of the evidence underpinning activity return, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and specific loading strategies is critical for the pursuit of favorable outcomes.
After a PRP injection, return to activity considerations should commensurate with the standard phases of healing. Using a progression from the acute to proliferative and remodeling phases as a guide is a reasonable approach given the known biological effects of PRP and expected clinical symptom sequelae. These stages, as well as the underlying injury and severity (e.g., tendinopathy versus tear), should dictate the nature of progression and expectations. This section presents an overview of progression from the acute to remodeling phase (Tables 1 and 2). Although evidence-based, variable protocols exist for managing individuals with tendinopathy following PRP. Unfortunately, there is an absence of literature regarding optimal programming for degenerative joint disease (e.g., OA) and discogenic pathology. Given the paucity of conclusive evidence for managing individuals after a PRP injection, information presented in this article is derived from previously published protocols (14,21,27,36,51,73,75), biological plausibility (based on known cellular responses to loading), as well as the authors’ clinical experience.
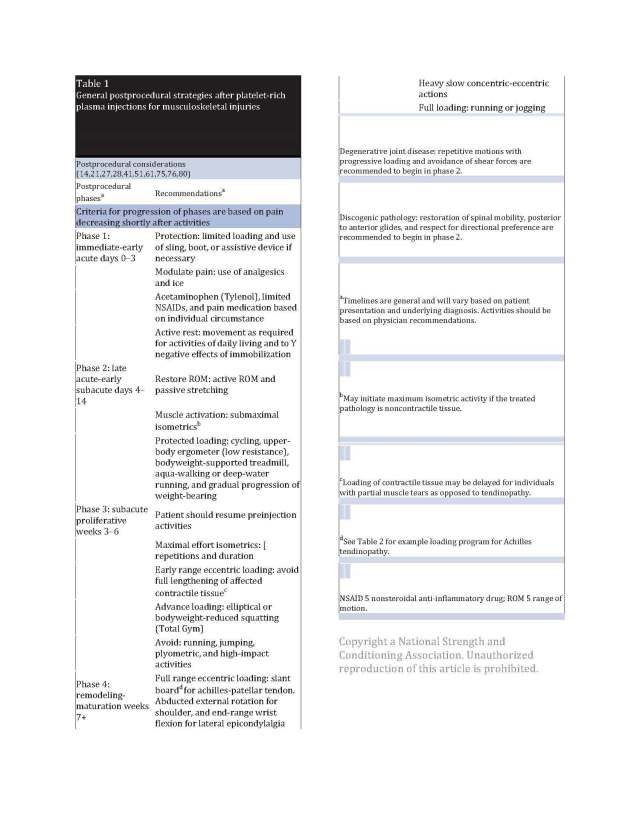

EARLY ACUTE STAGE (0–3 DAYS)
Immediately following a procedure, it is common to experience swelling, erythema, and increased pain (16). Furthermore, many individuals who receive a PRP injection have had clinical signs and symptoms for a variable period before the injection. Moreover, most individuals have been recalcitrant to previous conservative treatments. Thus, the patient will not be pain-free after the injection and may have increased pain and swelling until the potential PRP-induced inflammatory symptoms resolve.
At the early postprocedural stage, protection of the treated area, active rest, and management of the acute symptoms are the goal (41,61). Patients are encouraged to resume use of previously prescribed protective measures such as bracing or ambulatory aides. The decision to immobilize a joint is individualized and based on injury severity and location, as well as the patient’s typical activities of daily living.
In the early acute stage, the physician administering the PRP injection will provide specific instructions for immediate and early care. Given the absence of specific guidelines from the literature, material presented in this section will be largely drawn from the authors’ experience, individual outcome–based studies, biological evidence, and previously published narrative discussion articles (3,21,28,41,51,75,80). Nevertheless, the physician who provided the injection should be consulted before engaging in any activities at this stage.
Generally, in the early acute stage, the joint is protected for up to 3 days. Protection may be in the form of partial weight-bearing, use of sling or motion- restricted walking boot, or specific instructions to avoid use. Complete rest is discouraged, and active movements of the affected body part should be performed a few times a day to avoid the deleterious cellular effects of immobilization. The decision to limit weight-bearing is based on the patient’s presentation before the injection and the diagnosis. For example, a PRP injection to the plantar fascia may interfere with pain-free ambulation for a few days leading to the use of a restricted motion walking boot or partial weight-bearing. After the third day, a gradual increase in activity is encouraged, provided movements are tolerable (pain during movement that ceases after completion).
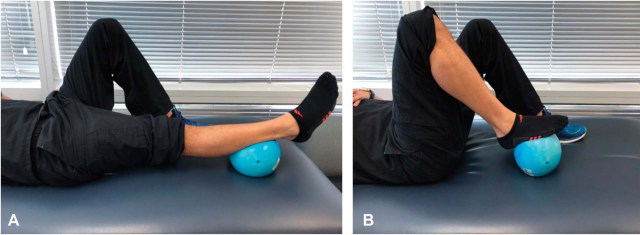
Figure 1.
Heel slide exercise. Individual is encouraged to repeatedly move the leg from extension (A) to flexion (B). Supine position eliminates gravity and use of ball eliminates friction.
Individuals with restricted weight-bearing are encouraged to progressively increase walking (duration and distance), and a progressive loading program is initiated. Failure to move the involved body part or weight-bear through the extremity may result in impairments that perpetuate or compound the pathology being treated. Similarly, overzealous activity will be counter- productive and may prolong the acute inflammatory phase.
During the early and late acute stages, patients may be provided with medications to reduce pain and interfere with inflammation. Although there is no consensus on the use of medication, approximately 42% of physicians limit the use of NSAIDs in some manner, despite limited evidence of these medications interfering with PRP out- comes (61,80). Alternative medications that may be used include narcotics (e.g., Percocet) and acetaminophen (e.g., Tylenol) (80). Corticosteroids are strictly discouraged because they are associated with reduced chondrocyte and tenocyte proliferation as well as cellular apoptosis (11,17,68). Furthermore, corticosteroids are likely to interfere with the inflammatory phase, which may preclude regeneration. Moreover, in vitro evidence suggests that adding a corticosteroid to a PRP preparation may reduce cellular proliferation (17). Regardless of the evidence, patients or clients should consult their physician before taking any medications. Cryotherapy (e.g., ice) may be used to reduce pain and inflammation; however, a concern is that ice may reduce platelet activation and interfere with the intended inflammatory cascade after PRP (61,80). Regarding ice, evidence suggests that hypothermia offers a protective effect of cartilage after trauma (76) and may reduce subacromial bursal thickness (72). Moreover, there is no published evidence that implicates ice as a factor that may hinder PRP outcomes. Given that no study has demonstrated an inferior result from ice and studies have used ice after a PRP injection with positive outcomes (51,75), there is no conclusive evidence to preclude use.
LATE ACUTE AND EARLY SUBACUTE PHASE (DAYS 4–14)
After the early protective stage, stretch- ing and isometrics are introduced at the affected regions. Isometrics have been shown to beneficially affect tendon healing, and evidence suggests that un- loading of muscle activity (through Botox) impairs the regenerative effects of platelets (44,83). In cases where contractile tissue is being treated (e.g., ten- don and muscle), the isometric efforts are submaximal. If the treated pathology is noncontractile tissue such as ligament or capsule, then the isometric contractions may be maximal effort. Isometrics should be repeated multiple times per day with 10-second holds working toward longer durations of up to 2 minutes by the subacute phase if tolerated. Activities such as cycling, deep-water running or water walking, and upper- body ergometry are introduced and advanced based on tolerance. In cases where a PRP injection is administered to treat OA, progressive and repetitive loading of the joint is encouraged. Activities such as low-resistance cycling and high-repetition heel slides (Figure 1) should be performed multiple times a day. A progressive increase in overall activities is pursued until the conclusion of week 2, when more pronounced advancements could be made. Before progression into the next phase, symptom tolerance to exercises must be gauged. The presence of some discomfort or pain during exercises is generally acceptable, provided symptom resolution occurs on completion. Pain or discomfort that occurs during exercises and remains elevated on completion suggests the need to evaluate and potentially regress programming.
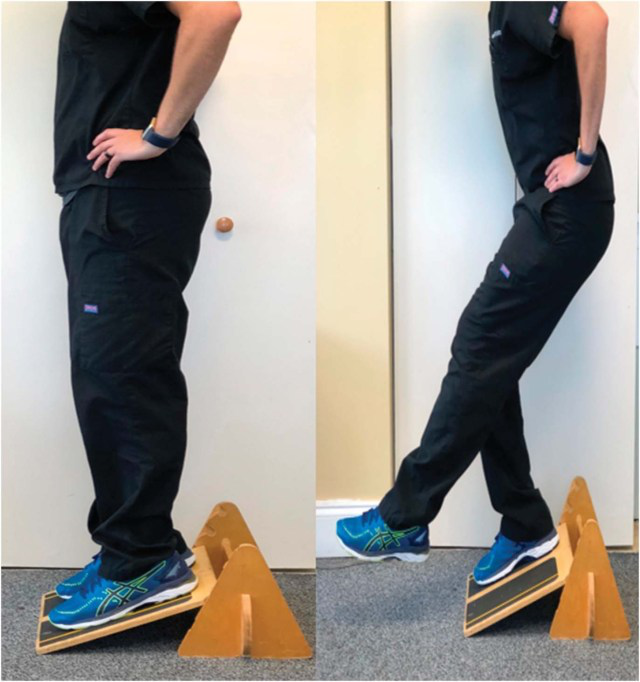
Figure 2.
Use of decline board to increase loading of patellar tendon during decline squatting. Note decline board places ankles in plantarflexion, which increases patellar tendon load through increased knee and ankle flexion from reduced tension in triceps surae musculature. Initial start position (left) and unilateral squatting (right). Bilateral lower extremities should be used to return from squat to start position (left).
SUBACUTE PHASE (WEEK 3–6)
The subacute phase is a key milestone, as this is where the pre-injection heterogeneity has a considerable influence. Specifically, the variability of clinical presentation before the PRP procedure will influence progression. Although those who were functioning at a high level (sport participation or weight lifting) before injection will be placed on a more progressive routine, others will follow a more conservative approach. In consideration of safety, a more conservative approach is presented.
The objective at this phase is to ensure that the cointroduction of mechanical forces is a primary component of the management strategy. The introduction of new exercises (and advancement of previously prescribed activities) that have been associated with regenerative changes in pathological tissue, upregulation of growth factors, and stimulation of cellular tissues is the overarching goal. Tables 1 and 2 highlight the associated exercises and progressions.
At week 3, isometric exercises are advanced to full effort for all conditions and increased hold times are recommended (full or reduced effort to failure if tolerated). Although isometrics may be considered easy for some, continuation is recommended, given the biological benefits (anabolic) previously discussed. Eccentric-focused muscle actions are first introduced within a limited tissue range and ultimately advanced to full range consistent with published protocols (27,51,73). Studies using eccentric loading after PRP range in performance frequency from 3 times a week to twice daily (2,19,21,26,51,73,79). The authors of this article generally base frequency on premorbid conditioning level with a frequency of 3 times a week up to once daily. Isotonic strengthening is introduced based on impairments, and joint loading is gradually increased from protected to full weight-bearing. An elliptical trainer may be used for lower extremity pathology, and squatting on an incline device to unload the joints is encouraged (e.g., Total Gym), particularly in cases of OA or cartilage pathology.
In some published investigations, individuals with focal tendinopathy or muscle strain have been progressed in a more aggressive manner after a PRP injection. For example, a case-control study of professional football players with a grade 2 (average grade) ham- string strain reported initiating a stretching program immediately and commencement of resistance training within the first week (75). In another study, patients with patellar tendinosis began a progressive eccentric loading program performed 3 times a week at the onset of week 2 (51). In the study, eccentric loading of the patellar tendon was gradually progressed with knee flexion limited to 458 for 1 week, which was progressed to 608 until week 6. At week 6, flexion was progressed to 908 with a decline board initiated shortly after- ward (Figure 2). In another study of patellar tendinopathy, athletic participants were allowed to resume premorbid activities within a week after their first PRP injection (32). Boesen et al. (14) initiated a twice-daily eccentric loading program and allowed 5 minutes of running within the first week after PRP injection for patients with Achilles tendinopathy. In contrast to the afore- mentioned studies, a case report of a patient with a partial distal triceps tear used 2 weeks of rest before any exercise-based intervention and initiated eccentric loading of the triceps at week 6 (21). The above studies high- light the variability of programs and the need to individualize progressions based on symptoms and underlying injury.
REMODELING PHASE (WEEK 7+)
The remodeling phase begins approximately at week 6 or 7. It is at this time when more advanced loading is initiated. Eccentric overload exercises are progressed to full range and heavy slow concentric actions are emphasized, with efforts to promote further soft-tissue remodeling. Although some examples of eccentric loading are provided in this article, readers may consult previously published articles in the Strength and Conditioning Journal for additional examples (18,58,50). Depending on the underlying injury, running is generally introduced, and individuals who are more active may be progressed to plyometric training with efforts to resume premorbid activities. Although running may be considered a risk factor for musculoskeletal conditions, much of this is speculative because recreational running is not a risk factor for lower extremity OA, and there is a body of evidence that it may positively increase the composition (e.g., hydration, proteoglycan content, and fiber hypertrophy) of the intervertebral disc (12,56). Individuals with OA are progressed to an appropriate exercise routine that pro- motes full range of motion with variable loading patterns based on clinical presentation. It is important to note that many of the activities introduced in this phase may be commenced earlier in athletic individuals who were functioning at a high level before injec- tion. In keeping with a “one size does not fit all” approach, individuals who were functioning at a much lower level (e.g., potential surgical candidate, use of assistive device for walking, or advanced degenerative disease) before injection may not reach more advanced performance milestones such as treadmill or plyometric training.
ADDITIONAL CONSIDERATIONS
The indications for a PRP injection are wide ranging and inclusive of OA, tendinopathy, cartilage defects, and muscle or tendon tears. Although each of these conditions (and the individual affected) have their own clinical presentation, degree of healing after the onset, and characteristic response to PRP, over- arching considerations exist and should be used to narrate the postprocedural phases. Musculoskeletal injuries or dis- orders present a constellation of signs and symptoms, with no paucity of evidence for rehabilitation or post rehabilitation guidelines because resumption of premorbid function is the goal. After a PRP injection, these goals remain; however, there are additional considerations for which this column sought to address, namely, the protective phase where pain and inflammation are ad- dressed, as well as the subacute and re- modeling phases where exercise principles are used to augment the effects of PRP.
NONSTEROIDAL ANTI- INFLAMMATORY DRUGS
In the early acute phase, evidence from numerous studies and narrative articles suggest a short period of pain and inflammation. Although there is a consensus for a few days of rest, variability exists regarding the use of NSAIDs. The concern for NSAID use after PRP is based on reduced platelet aggregation, impaired growth factor release, interruption of the intended inflammation, and deleterious cellular effects. Regarding cellular function, the evidence is divergent. In one study of individuals exposed to a prolonged bout of running, NSAID use abolished the adaptive rise in collagen synthesis seen in the subjects who took a placebo (22). In another study, NSAIDs were infused into the quadriceps of subjects before, during, and for 4.5 hours after an eccentric training bout (63). Results indicated that the NSAIDs prevented satellite cell activity in the infused quadriceps, whereas the control extremity showed a 96% increase in activity. In contrast to the aforementioned study, Mackey et al. (60) studied the effect of NSAID ingestion on satellite cell activity of the quadriceps muscle (through biopsy) before and after (up to 30 days) a laboratory- induced muscle injury. In the study, NSAID consumption was associated with increased satellite cell activation compared with placebo, suggesting that NSAIDs may have a different effect on injured versus healthy muscle (60).
Additional evidence on the effect of NSAIDs may come from clinical studies. In one study, individuals with chronic Achilles tendinopathy were randomized to 1 week of NSAIDs or placebo (42). Results from biopsy and ultrasound indicate that ibuprofen had no effect on collagen expression and growth factors. Dideriksen et al. (30) studied the effects of NSAID use on patellar tendons of elderly adults who were immobilized for 2 weeks. Although collagen synthesis was decreased in the immobilized limbs as expected, the NSAID group revealed no difference when compared with pla- cebo. Although evidence supports the premise that nonselective NSAIDs (e.g., naproxen) may interfere with platelet aggregation (55), growth factor levels in PRP are not altered (59,82). In conclusion, NSAID use does not compromise growth factor release and is more likely to have a deleterious effect on healthy structures as opposed to patho- logical tissues. Moreover, other medication classes such as acetaminophen and select opioid drugs may negatively influence post exercise protein synthesis and produce in vitro chondrotoxicity, respectively (1,81). Given the potential for NSAIDs to reduce pain and inflammation and the potential of other medication classes to negatively influence cells, it seems there is little downside to intermittent, low-dose use in the early acute phase.
MECHANOBIOLOGY
Formal postprocedural management strategies have been documented in the literature after PRP injection; how- ever, these studies are primarily limited to tendon pathology and muscle strains (14,21,27,36,51,73,75). Although variability exists with respect to exercise selection, progressions, and inclusion time, a majority of programming includes the introduction of mechanical forces designed to work in synergy with respect to PRP. Specifically, most programs focus on initiating early active movement to combat the deleterious effects of immobilization, followed by progressive mechanical loading of the tissues. Progressive mechanical loading (referred to as mechanotherapy) has been shown to pro- duce a favorable anabolic environment and promote tissue regeneration. Understanding the mechanical stimuli to which musculoskeletal cells best respond and the mechanisms these cells use to convert mechanical signals into a cellular response is required to effectively introduce interventions that function synergistically with PRP.
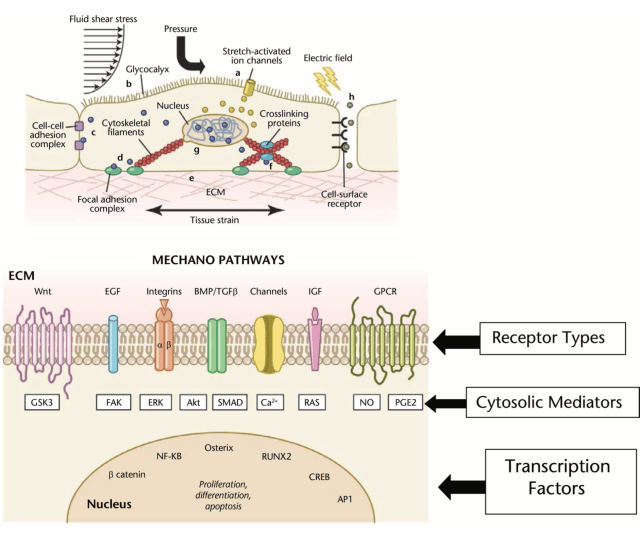
A brief, albeit important discussion of the concept of mechanotransduction is necessary to appreciate the benefits associated with mechanical loading. In general, when joints move, mechanical energy is added to an existent equilibrium of the body and stress is introduced to the load-bearing tissues (48). Each muscle, tendon, and bone is composed of cells that are linked together through an extracellular matrix. Cells adhere to these extracellular matrix scaffolds (composed of collagen, glycoproteins, and proteoglycans) through binding of specific receptors of the cell surface (48). Integrins span the cell surface membrane and communicate signals or stress from the external to the internal environment. Essentially, integrins serve as mechanoreceptors given they are the first molecules on the cell surface to sense a mechanical signal (e.g., compression, tension, etc.) and transmit it across the membrane. These signals can alter gene expression, protein synthesis, and metabolism in a manner similar to hormones, growth factors, and cytokines (48).
Mechanotransduction is the process by which the tissues undergo an adaptive structural change in response to a mechanical load (53). With mechanotransduction, efforts aim to convert potentially destructive mechanical ef- fects into constructive events that promote recovery through adaptation at the vicinity of the “stressed” cell or neighboring cells (47). Similar to bone getting stronger in response to weight- bearing (another form of mechano- transduction), tendon or muscle would remodel in response to loading. Mechanotransduction follows a sequence of 3 steps, which include mechanocoupling, cell-to-cell communication (passing of loading message from one area of tendon to another), and a cellular response such as collagen synthesis or chondrogenesis (53).
A necessary requisite for a cellular response is the presence of an appropriate load to the tissue of interest. The physical load that induces mechano- transduction is referred to as mechano- coupling (53). A key point with mechanocoupling is that the “overload” needs to be appropriate, progressive, and short of the point where injury risk presents itself. For example, eccentrically overloading (mechanocoupling) an involved tendon sets off the remaining physical events of mechanotransduction through stimulation of the tendon cell (tenocyte), which in turn leads to cell- to-cell communication and a cellular response (collagen synthesis). A detailed discussion of mechanotransduction with illustrations of the cellular processes described can be found in the article by Khan and Scott (53).
In summary, for mechanotransduction to occur, a load is applied to the region of interest. This load, through integrins, communicates the load’s message to the cell where the DNA and cytoplasmic elements are located. When the signal of a “load” is received at the cell, a message is communicated to the cell’s nucleus (DNA). Once the nucleus receives the signal, the messenger RNA transcribes the message and shuttles it to the cytoplasm where it is translated into a regenerative response such as collagen synthesis, which would be incorporated into the cellular matrix.
The more common exercise principles used to promote mechanotransduction after PRP include active and passive movements, which primarily serve to prevent the deleterious effects of immobilization (30,69,77) and muscle performance activities inclusive of iso- metric contractions, eccentric over- load, and heavy slow resistance training. Fortunately, the mechanical load on the tissues from both isometric and eccentric training induces other positive anabolic responses that further support the effects of PRP in promoting a regenerative environment.
MOVEMENT AND MOBILIZATION
Active and passive motion should be introduced in the early acute phase to deter the negative effects of immobilization. Aside from mitigating movement impairments such as arthrofibrosis, repetitive movements have a favorable effect on cartilage and the intervertebral disc. Unfortunately, much of what we have gleaned in the area of articular cartilage is based on studies investigating the effects of continuous passive movement (CPM) versus immobilization. Nevertheless, these studies provide an under- standing of the biophysiological effects that steer early movement and avoid- ance of prolonged immobilization. Specifically, the biological evidence has shown that integrins in diseased human chondrocytes are sensitive to mechanical stimulation and increase expression of type 2 collagen and proteoglycans (49). In support of these findings, an in vitro study of human arthritic chondrocytes determined that repeated compression influenced chondrogenesis and reduced catabolic events through downregulation of collagenase expression (29). A key point here is that arthritic chondrocytes are characterized by a catabolic phenotype and a repeated compression model downregulated this environment (29). Furthermore, a greater understanding may be gleaned from in vivo laboratory studies of nonhuman knees. In a con- trolled laboratory study of rabbit knees, the anterior cruciate ligament was resected and the knee joint lines were evaluated 4 weeks after surgery. During the 4 weeks, the rabbits were allocated to CPM, treadmill, or sedentary environments. After 4 weeks, the CPM group had normal articular cartilage, whereas the treadmill and sedentary groups experienced surface abrasion. In addition, the CPM group had lower levels of inflammatory cytokines and a normal level of chondrocytes without damaged collagen fibers when com- pared with the other groups that had chondrocyte apoptosis, reduced cartilage thickness, and collagen damage (20). Shimizu et al. (77) investigated the repair response of cartilage defects in rabbit knee models and found that joints exposed to CPM had higher chondrocyte numbers than immobilization groups. In addition, delaying CPM for 1 week significantly reduced the beneficial effects on cartilage. Although the aforementioned in vivo studies further highlight the effects of activity and inactivity, one must be cautious in the generalization of these findings to the human clinical environment.
Regarding the lumbar intervertebral disc, a body of evidence has suggested that increased diffusion of water con- tent into the disc is associated with pain reduction among individuals with low back pain (8–10). Studies have shown that, when appropriate, posterior to anterior joint mobilization (non- thrust) and prone extension exercises may increase water content within the disc and subsequently be associated with reduced pain (8,10). Assuming that these interventions are appropriate to the individual’s diagnosis, they may be considered as part of routine care.
ISOMETRIC TRAINING
Although the clinical research on isometric training is limited, evidence does suggest a beneficial response for acute tendinopathy (72). In particular, evidence from one investigation (3-arm [group] trial) has indicated that isometric strengthening produced significant improvements in pain and function as well as reduced tendon thickening (observed for 71% of participants albeit not statistically significant) after inter- vention (72). Unfortunately, the details of the isometric dosing were unclear, as the authors reported progressing from 3 to 5 times per day with the duration of contraction progressing from 10 to 20 seconds with no mention of repetitions performed with each session. Research on pain ratings during contraction and pressure pain thresholds (sensitivity to pressure) have been favorable regarding isometric training; however, the use of healthy adults in these studies certainly skews the clinical value (46,57). From a dosing perspective, evidence suggests that submaximal isometric contractions are best held until failure, whereas maximal effort contractions may be held for durations as short as 5 seconds (39,43,44,46,57).
Regarding anabolic hormones, growth factors, and cytokines, isometric training has been shown to produce increases in growth hormone, testosterone, and IGF (IGF1Ea and MGF) and decreases in myostatin (37,39,43,44). In the study by Hakkinen et al. (39), repeated maximum effort 5-second isometric contractions were used and although favorable changes were noted in all subjects, younger men had a greater response than older men.
ECCENTRIC LOADING
A compelling body of evidence exists to support the inclusion of both isometric and eccentric overload training. This evidence is in the form of isolated studies showing a regenerative effect from eccentric training on tendinopathy (23,71) as well as additional studies indicating a favorable anabolic response (hormones and growth factors) and reduced pain when compared with concentric-based training (43,44,54,74). Furthermore, many published PRP studies have incorporated both isometric and eccentric overload training with favorable outcomes (14,21,27,51).
Heel drops are a frequently prescribed eccentric exercise for Achilles tendinopathy. A program of twice-daily eccentric overload exercises (Figure 3) resulted in cellular matrix remodeling and reversal of degenerative changes among individuals with Achilles tendinopathy (71). In another investigation of Achilles tendinopathy, the effect of adding PRP to a pro- gram of eccentric overload activity was evaluated (27). In the study, both groups showed considerable improvement; however, the addition of a PRP injection offered no additional benefit. In contrast to the aforementioned results, Boesen et al. (14) studied the effect of eccentric training alone versus eccentric with PRP to individuals with Achilles tendinopathy and found that PRP plus eccentric over- load has superior outcomes regarding tendon structure and pain reduction when compared with eccentric alone. In another study, heavy slow resistance training offered a comparable benefit (tendon structure and clinical outcomes) to eccentric loading, thus may serve as a valuable alternative or be prescribed concurrently (13).
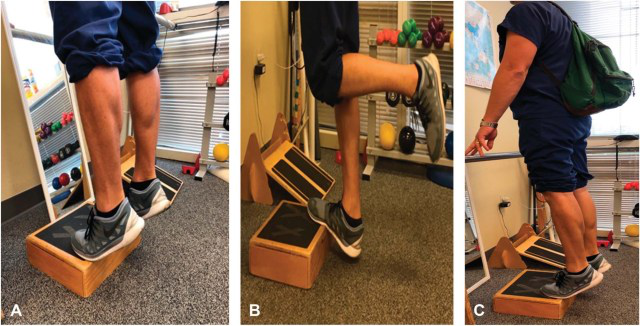
Figure 3.
Eccentric loading for Achilles tendinopathy. (A) Initial movement into plantarflexion bilaterally off step and (B) lowering on the affected leg only to end-range dorsiflexion. Knee is in extension. May be performed with slight knee flexion also in accordance with evidence. (C) Backpack loading progression.
In addition to the clinical benefits and regenerative effects of eccentric overload training on tendinopathy, a considerable body of evidence has identified favorable anabolic growth factor responses. Eccentric overload activity has been shown to induce autocrine and paracrine IGF responses (both IGF-1Ea and mechano- growth factor [IGF-1Ec]) in tendon and muscle (38,40,43,44), which further supports and influences the desired matrix remodeling effect from PRP. Other benefits of eccentric overload include myostatin inhibition (44). Myo- statin is a myokine (cytokine) found in muscle tissue that alters muscle cell differentiation in favor of scar-forming myofibroblasts and has an inhibitory effect on satellite cell activation, hypertrophy, and protein synthesis (15,38). Finally, evidence suggest that repeated bouts of eccentric overload exercise promote a reduction in the inflammatory cytokines (TNF-a and interleukin 8) and an increase in interleukin-10 (anti-inflammatory cytokine) (45).
The most appropriate dosage (frequency and repetition range) for eccentric training has yet to be determined (67); however, the protocol that is often followed after a PRP injection was developed by Alfred- son et al. (2,14,71). Eccentric overload exercises, using the Alfredson protocol, are performed for 3 sets of 15 repetitions, twice daily, 7 days per week, for 12–24 weeks (2). This protocol has resulted in increased peak torque, decreased pain, and a return to previous level of function in addition to imaging confirmed tendon regeneration (2,71). Recent investigations found that performance of such exercises once daily or 3 times a week may offer similar benefits to twice daily regarding tendinopathy (19,52). Moreover, Stevens et al. (79) found that with respect to clinical outcomes, a “do as tolerated” program revealed comparable clinical outcomes; however, structural changes were not assessed.
CONCLUSION
Regenerative medicine is a growing field of medicine with rapidly expanding indi- cations and widespread use. Sports medicine and rehabilitation professionals will contend with individuals who have had these procedures. An understanding of the treating physician’s recommendations, basic science, clinical evidence, and postprocedural precautions is necessary for safely reintegrating exercise program- ming. Although PRP and targeted exercise strategies share common goals of promoting an anabolic or regenerative environment, individuals receiving these procedures are contending with an injury or disorder that requires safety considerations. Specifically, a short period of active rest with the use of ice and NSAIDs is not precluded during the acute postprocedural stage. After the acute period, an ideal microenvironment that promotes appropriately timed movements with progressive overload will seemingly enhance functional outcomes and augment the regenerative response from PRP. Research into postprocedural management is still in its infancy and there is a need for an improved understanding of how mechanical forces may be used to optimize outcomes following PRP procedures.
KEY POINTS
- Postprocedural guidelines vary and are usually based on the individual’s clinical presentation and premorbid activity level.
- A brief period of active rest is recommended following the procedure.
- Early initiation of movement is critical to mitigate the negative effects of immobilization.
- The use of NSAIDs and ice is generally safe in the acute and early sub-acute phases.
- Mechanical loading is essential and promotes a favorable microenvironment for PRP.
Conflicts of Interest and Source of Funding: The authors report no conflicts of interest and no source of funding.
Morey J. Kolber is a Professor at Nova Southeastern University in the Department of Physical Therapy.
Joseph Purita is the Medical Director and an Orthopaedic Surgeon at the Institute of Regenerative Medicine.
Christian Paulus is the Executive Director at the American Academy of Regenerative Medicine.
Jeremy A. Carreno is the Chief Clinical Biological Specialist at the Institute of Regenerative Medicine.
William J. Hanney is an Assistant Professor at the University of Central Florida in the Department of Health Professions.
References
1. Abrams GD, Chang W, and Dragoo JL. In vitro chondrotoxicity of nonsteroidal anti- inflammatory drugs and opioid medications. Am J Sports Med 45: 3345–3350, 2017.
2. Alfredson H, Pietila T, Jonsson P, and Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med 26: 360–366, 1998.
3. Ambrosio F, Wolf SL, Delitto A, Fitzgerald GK, Badylak SF, Boninger ML, and Russell AJ. The emerging relationship between regenerative medicine and physical therapeutics. Phys Ther 90: 1807–1814, 2010.
4. Andia I and Maffulli N. Biological therapies in regenerative sports medicine. Sports Med 47: 807–828, 2017.
5. Andia I, Sanchez M, and Maffulli N. Basic science: Molecular and biological aspects of platelet-rich plasma therapies. Oper Tech Orthop 22: 3–9, 2012.
6. Baksh N, Hannon CP, Murawski CD, Smyth NA, and Kennedy JG. Platelet-rich plasma in tendon models: A systematic review of basic science literature. Arthroscopy 29: 596–607, 2013.
7. Basso M, Cavagnaro L, Zanirato A, Divano S, Formica C, Formica M, and Felli L. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet Surg 101: 93–104, 2017.
8. Beattie PF, Arnot CF, Donley JW, Noda H, and Bailey L. The immediate reduction in low back pain intensity following lumbar joint mobilization and prone press-ups is associated with increased diffusion of water in the L5-S1 intervertebral disc. J Orthop Sports Phys Ther 40: 256–264, 2010.
9. Beattie PF, Butts R, Donley JW, and Liuzzo DM. The within-session change in low back pain intensity following spinal manipulative therapy is related to differences in diffusion of water in the intervertebral discs of the upper lumbar spine and L5-S1. J Orthop Sports Phys Ther 44: 19–29, 2014.
10. Beattie PF, Donley JW, Arnot CF, and Miller R. The change in the diffusion of water in normal and degenerative lumbar intervertebral discs following joint mobilization compared to prone lying. J Orthop Sports Phys Ther 39: 4–11, 2009.
11. Beitzel K, McCarthy MB, Cote MP, Apostolakos J, Russell RP, Bradley J, ElAttrache NS, Romeo AA, Arciero RA, and Mazzocca AD. The effect of ketorolac tromethamine, methylprednisolone, and platelet-rich plasma on human chondrocyte and tenocyte viability. Arthroscopy 29: 1164–1174, 2013.
12. Belavy DL, Quittner MJ, Ridgers N, Ling Y, Connell D, and Rantalainen T. Running exercise strengthens the intervertebral disc. Sci Rep 7: 1–8, 2017.
13. Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, and Magnusson SP. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: A randomized controlled trial. Am J Sports Med 43: 1704–1711, 2015.
14. Boesen AP, Hansen R, Boesen MI, Malliaras P, and Langberg H. Effect of high- volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: A randomized double-blinded prospective study. Am J Sports Med 45: 2034–2043, 2017.
15. Burks TN and Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet Muscle 1: 19, 2011.
16. Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR Jr, and Cole BJ. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 31: 2213–2221, 2015.
17. Carofino B, Chowaniec DM, McCarthy MB, Bradley JP, Delaronde S, Beitzel K, Cote MP, Arciero RA, and Mazzocca AD. Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: An in vitro study on human tendon cells. Arthroscopy 28: 711–719, 2012.
18. Chaconas E and Kolber M. Eccentric training for the shoulder external rotators part 2: Practical applications. Strength Cond J 35: 8–10, 2013.
19. Chaconas EJ, Kolber MJ, Hanney WJ, Daugherty ML, Wilson SH, and Sheets C. Shoulder external rotator eccentric training versus general shoulder exercise for subacromial pain syndrome: A randomized controlled trial. Int J Sports Phys Ther 12: 1121–1133, 2017.
20. Chang NJ, Lee KW, Chu CJ, Shie MY, Chou PH, Lin CC, and Liang PI. Preclinical assessment of early continuous passive motion and treadmill therapeutic exercises for generating chondroprotective effects after anterior cruciate ligament rupture. Am J Sports Med 45: 2284–2293, 2017.
21. Cheatham SW, Kolber MJ, Salamh PA, and Hanney WJ. Rehabilitation of a partially torn distal triceps tendon after platelet rich plasma injection: A case report. Int J Sports Phys Ther 8: 290–299, 2013.
22. Christensen B, Dandanell S, Kjaer M, and Langberg H. Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J Appl Physiol 110: 137–141, 2011.
23. Croisier JL, Foidart-Dessalle M, Tinant F, Crielaard JM, and Forthomme B. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med 41: 269– 275, 2007.
24. Dai WL, Zhou AG, Zhang H, and Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: A meta- analysis of randomized controlled trials. Arthroscopy 33: 659–670, 2017.
25. de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, and van Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 36: 1171–1178, 2008.
26. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, and van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med 45: 387–392, 2011.
27. de Vos RJ, Weir A, van Schie HT, Bierma- Zeinstra SM, Verhaar JA, Weinans H, and Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA 303: 144–149, 2010.
28. Deal JB, Smith E, Heard W, O’Brien MJ, and Savoie FH III. Platelet-rich plasma for primary treatment of partial ulnar collateral ligament tears: MRI correlation with results. Orthop J Sports Med 5: 2325967117738238, 2017.
29. Diao HJ, Fung HS, Yeung P, Lam KL, Yan CH, and Chan BP. Dynamic cyclic compression modulates the chondrogenic phenotype in human chondrocytes from late stage osteoarthritis. Biochem Biophys Res Commun 486: 14–21, 2017.
30. Dideriksen K, Boesen AP, Reitelseder S, Couppe C, Svensson R, Schjerling P, Magnusson SP, Holm L, and Kjaer M. Tendon collagen synthesis declines with immobilization in elderly humans: No effect of anti-inflammatory medication. J Appl Physiol 122: 273–282, 2017.
31. Dupley L and Charalambous CP. Platelet- rich plasma injections as a treatment for refractory patellar tendinosis: A meta- analysis of randomised trials. Knee Surg Relat Res 29: 165–171, 2017.
32. Emerson B, Tabor M, Liggins C, Huff L, and Kolber MJ. The efficacy of platelet rich plasma as an intervention for patellar tendinopathy: A case series. Clin J Sports Med 26: e30–e31, 2016.
33. Fitzpatrick J, Bulsara M, and Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: A meta-analysis of randomized controlled clinical trials. Am J Sports Med 45: 226–233, 2017.
34. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, and Rodeo SA. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med 37: 2259– 2272, 2009.
35. Ghodasra JH, Wang D, Jayakar RG, Jensen AR, Yamaguchi KT, Hegde VV, and Jones KJ. The assessment of quality, accuracy, and readability of online educational resources for platelet-rich plasma. Arthroscopy 34: 272–278, 2018.
36. Gosens T, Den Oudsten BL, Fievez E, van ’t Spijker P, and Fievez A. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: A prospective cohort study and the influence of previous treatments. Int Orthop 36: 1941–1946, 2012.
37. Greig CA, Hameed M, Young A, Goldspink G, and Noble B. Skeletal muscle IGF-I isoform expression in healthy women after isometric exercise. Growth Horm IGF Res 16: 373–376, 2006.
38. Haddad F and Adams GR. Selected contribution: Acute cellular and molecular responses to resistance exercise. J Appl Physiol 93: 394–403, 2002.
39. Hakkinen K, Pakarinen A, Newton RU, and Kraemer WJ. Acute hormone responses to heavy resistance lower and upper extremity exercise in young versus old men. Eur J Appl Physiol Occup Physiol 77: 312–319, 1998.
40. Hameed M, Toft AD, Pedersen BK, Harridge SD, and Goldspink G. Effects of eccentric cycling exercise on IGF-I splice variant expression in the muscles of young and elderly people. Scand J Med Sci Sports 18: 447–452, 2008.
41. Head PL. Rehabilitation considerations in regenerative medicine. Phys Med Rehabil Clin N Am 27: 1043–1054, 2016.
42. Heinemeier KM, Ohlenschlaeger TF, Mikkelsen UR, Sonder F, Schjerling P, Svensson RB, and Kjaer M. Effects of anti- inflammatory (NSAID) treatment on human tendinopathic tissue. J Appl Physiol (1985) 123: 1397–1405, 2017.
43. Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, and Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol 582: 1303– 1316, 2007.
44. Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, and Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: Differential effects of specific contraction types. J Appl Physiol (1985) 102: 573–581, 2007.
45. Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, and Suzuki K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev 10: 75–90, 2004.
46. Hoeger Bement MK, Dicapo J, Rasiarmos R, and Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc 40: 1880–1889, 2008.
47. Huang C, Holfeld J, Schaden W, Orgill D, and Ogawa R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med 19: 555–564, 2013.
48. Ingber DE. Tensegrity and mechanotransduction. J Bodyw Mov Ther 12: 198–200, 2008.
49. Jeong JY, Park SH, Shin JW, Kang YG, Han KH, and Shin JW. Effects of intermittent hydrostatic pressure magnitude on the chondrogenesis of MSCs without biochemical agents under 3D co-culture. J Mater Sci Mater Med 23: 2773–2781, 2012.
50. Kaplan K, Hanney W, Cheatham S, Masaracchio M, Liu A, and Kolber M. Rotator cuff tendinopathy: An evidence-based overview for the sports medicine professional. Strength Cond J 40: 61–70, 2018.
51. Kaux JF, Forthomme B, Namurois MH, Bauvir P, Defawe N, Delvaux F, Lehance C, Crielaard JM, and Croisier JL. Description of a standardized rehabilitation program based on sub-maximal eccentric following a platelet-rich plasma infiltration for jumper’s knee. Muscles Ligaments Tendons J 4: 85–89, 2014.
52. Kelc R, Trapecar M, Gradisnik L, Rupnik MS, and Vogrin M. Platelet-rich plasma, especially when combined with a TGF-beta inhibitor promotes proliferation, viability and myogenic differentiation of myoblasts in vitro. PLoS One 10: e0117302, 2015.
53. Khan KM and Scott A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br J Sports Med 43: 247–252, 2009.
54. Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, and Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 19: 500–510, 2009.
55. Knijff-Dutmer EA, Kalsbeek-Batenburg EM, Koerts J, and van de Laar MA. Platelet function is inhibited by non-selective non- steroidal anti-inflammatory drugs but not by cyclo-oxygenase-2-selective inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford) 41: 458–461, 2002.
56. Kolber MJ, Hanney WJ, Cheatham SW, and Salamh PA. Risk factors for hip osteoarthritis: Insight for the strength and conditioning professional. Strength Cond J 39: 35–41, 2017.
57. Lemley KJ, Drewek B, Hunter SK, and Hoeger Bement MK. Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc 46: 185–191, 2014.
58. Lorenz D. Eccentric exercise interventions for tendinopathy. Strength Cond J 32: 90– 98, 2010.
59. Ludwig HC, Birdwhistell KE, Brainard BM, and Franklin SP. Use of a cyclooxygenase- 2 inhibitor does not inhibit platelet activation or growth factor release from platelet-rich plasma. Am J Sports Med 45: 3351–3357, 2017.
60. Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, Aagaard P, Durigan JL, and Kjaer M. Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti- inflammatory medication. FASEB J 30: 2266–2281, 2016.
61. Mautner K, Malanga G, and Colberg R. Optimization of ingredients, procedures and rehabilitation for platelet-rich plasma injections for chronic tendinopathy. Pain Manag 1: 523–532, 2011.
62. McClure MJ, Garg K, Simpson DG, Ryan JJ, Sell SA, Bowlin GL, and Ericksen JJ. The influence of platelet-rich plasma on myogenic differentiation. J Tissue Eng Regen Med 10: E239–E249, 2016.
63. Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, and Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107: 1600–1611, 2009.
64. Mishra A, Harmon K, Woodall J, and Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol 13: 1185–1195, 2012.
65. Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, and Jacobs CR. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 15: 431–435, 2009.
66. Moussa M, Lajeunesse D, Hilal G, El Atat O, Haykal G, Serhal R, Chalhoub A, Khalil C, and Alaaeddine N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res 352: 146–156, 2017.
67. Murtaugh B and Ihm JM. Eccentric training for the treatment of tendinopathies. Curr Sports Med Rep 12: 175–182, 2013.
68. Muto T, Kokubu T, Mifune Y, Inui A, Sakata R, Harada Y, Takase F, and Kurosaka M. Effects of platelet-rich plasma and triamcinolone acetonide on interleukin-1ss- stimulated human rotator cuff-derived cells. Bone Joint Res 5: 602–609, 2016.
69. Mutsuzaki H, Nakajima H, Wadano Y, Furuhata S, and Sakane M. Influence of knee immobilization on chondrocyte apoptosis and histological features of the anterior cruciate ligament insertion and articular cartilage in rabbits. Int J Mol Sci 18: E253, 2017.
70. Navani A, Li G, and Chrystal J. Platelet rich plasma in musculoskeletal pathology: A necessary rescue or a lost cause? Pain Physician 20: E345–E356, 2017.
71. Ohberg L, Lorentzon R, and Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: Normalised tendon structure and decreased thickness at follow up. Br J Sports Med 38: 8–11, 2004.
72. Parle PJ, Riddiford-Harland DL, Howitt CD, and Lewis JS. Acute rotator cuff tendinopathy: Does ice, low load isometric exercise, or a combination of the two produce an analgaesic effect? Br J Sports Med 51: 208–209, 2017.
73. Peerbooms JC, Sluimer J, Bruijn DJ, and Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: Platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 38: 255–262, 2010.
74. Peterson M, Butler S, Eriksson M, and Svardsudd K. A randomized controlled trial of eccentric vs. concentric graded exercise in chronic tennis elbow (lateral elbow tendino- pathy). Clin Rehabil 28: 862–872, 2014.
75. Rettig AC, Meyer S, and Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med 1: 2325967113494354, 2013.
76. Riegger J, Zimmermann M, Joos H, Kappe T, and Brenner RE. Hypothermia promotes cell-protective and chondroprotective effects after blunt cartilage trauma. Am J Sports Med 46: 420–430, 2018.
77. Shimizu T, Videman T, Shimazaki K, and Mooney V. Experimental study on the repair of full thickness articular cartilage defects: Effects of varying periods of continuous passive motion, cage activity, and immobilization. J Orthop Res 5: 187–197, 1987.
78. Singla V, Batra YK, Bharti N, Goni VG, and Marwaha N. Steroid vs. platelet-rich plasma in ultrasound-guided sacroiliac joint injection for chronic low back pain. Pain Pract 17: 782–791, 2017.
79. Stevens M and Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: A randomized controlled trial. J Orthop Sports Phys Ther 44: 59–67, 2014.
80. Sussman WI, Mautner K, and Malanga G. The role of rehabilitation after regenerative and orthobiologic procedures for the treatment of tendinopathy: A systematic review. Regen Med 13: 249–263, 2018.
81. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, and Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002.
82. Utku B, Donmez G, Erisgen G, Akin S, Demirel HA, Korkusuz F, and Doral MN. Meloxicam and diclofenac do not change VEGF and PDGF-ABserum levels of platelet-rich plasma. Turk J Med Sci 47: 570–576, 2017.
83. Virchenko O and Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 77: 806–812, 2006.
84. Wu PI, Diaz R, and Borg-Stein J. Platelet- rich plasma. Phys Med Rehabil Clin N Am 27: 825–853, 2016.
85. Yang WY, Han YH, Cao XW, Pan JK, Zeng LF, Lin JT, and Liu J. Platelet-rich plasma as a treatment for plantar fasciitis: A meta- analysis of randomized controlled trials. Medicine 96: e8475, 2017.
86. Zhang JY, Fabricant PD, Ishmael CR, Wang JC, Petrigliano FA, and Jones KJ. Utilization of platelet-rich plasma for musculoskeletal injuries: An analysis of current treatment trends in the United States. Orthop J Sports Med 4: 2325967116676241, 2016.
87. Zhou Y and Wang JH. PRP treatment efficacy for tendinopathy: A review of basic science studies. Biomed Res Int 2016: 9103792, 2016.
88. Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, and Peng J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis Cartilage 21: 1627–1637, 2013.
Source URL: https://stemcellorthopedic.com/physical-therapy-after-stem-cell-therapy/
